CRF researcher Leonid Sheps has developed a new spectroscopic method, Time-Resolved Broadband Cavity-Enhanced Absorption Spectroscopy (TR-BB-CEAS). The new technique is capable of following the time evolution of transient intermediates as they are produced and consumed in a gas-phase chemical reaction by monitoring their absorption of near-UV to visible light (300–700 nm).
Existing absorption techniques can effectively hone in on some chemical intermediates, one at a time, by using single-wavelength detection. However, the ability to simultaneously probe many species across a broad spectral range with fast time resolution has eluded researchers. Such multiplexed detection is particularly challenging in gas-phase reactions because the sample densities (and, consequently, absorption signal strengths) are much lower in gases than in liquids. Yet, uncovering the behavior of these short-lived, highly dilute intermediates is critical because these species frequently govern the outcomes of the chemical reactions being studied. In combustion research, for example, characterizing reaction intermediates can help guide efforts to optimize engines for increased efficiency, reduced pollution, and the ability to run on alternative fuels.
Aware of this need, Leonid sought to create a new tool that met four specific criteria. “The ideal system for this purpose would be multiplexed, that is, able to illuminate multiple intermediates at once, and have microsecond time resolution,” says Leonid. “Other design goals were to create a system that was nonintrusive, so it wouldn’t interfere with the reactions, and highly sensitive in order to capture important species, even if present in low concentrations.”
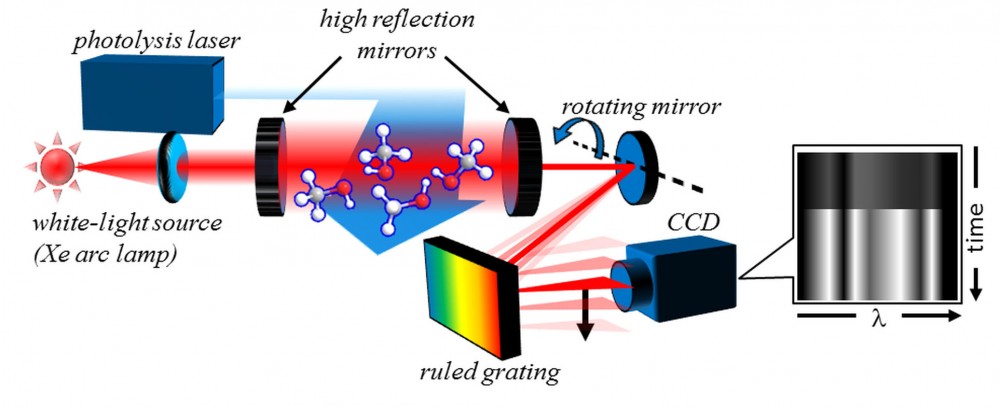
Leonid succeeded in meeting not only these criteria, but the further objectives of creating a robust, simple-to-use, and economical experimental apparatus. Shown in Figure 1, TR-BB-CEAS is based on a broadband optical resonator cavity filled by a slowly flowing sample gas mixture. A simple xenon lamp provides broadband (white) probe light, which is directed into the cavity. At each end of the cavity are highly reflective (99.5% reflective) broadband mirrors that enable the broad spectrum of the probe light to travel back and forth for many passes within the chamber. This results in long effective path lengths, increasing the sensitivity of the system to the absorption of the light by dilute transient species. A photolysis laser pulse triggers the chemical reaction at a set time, and subsequent changes in the absorption spectrum track the reaction’s progress.

Figure 1. TR-BB-CEAS experimental setup, which includes a white-light probe source, a broadband optical cavity integrated with a photolytic-initiation chemical reactor, and a time-resolved spectrometer.
To record the entire absorption spectrum evolution in real-time, Leonid built a novel time-resolved spectrometer. In this system, a ruled optical grating disperses the spectral information of light exiting the cavity along the x axis (the rows) of a detector—a charge-coupled device (CCD) camera. At the same time, a precisely controlled movable mirror quickly sweeps the output spectrum along the camera’s y axis (the columns). These two actions create a two-dimensional image—a spatial map of the transient absorption, as shown on the right side of Figure 1. “From this map, we can chart the appearance and disappearance of various species, even if their lifetimes are extremely short—only a few millionths of a second—which greatly increases our understanding of very rapid chemical reactions,” says Leonid.
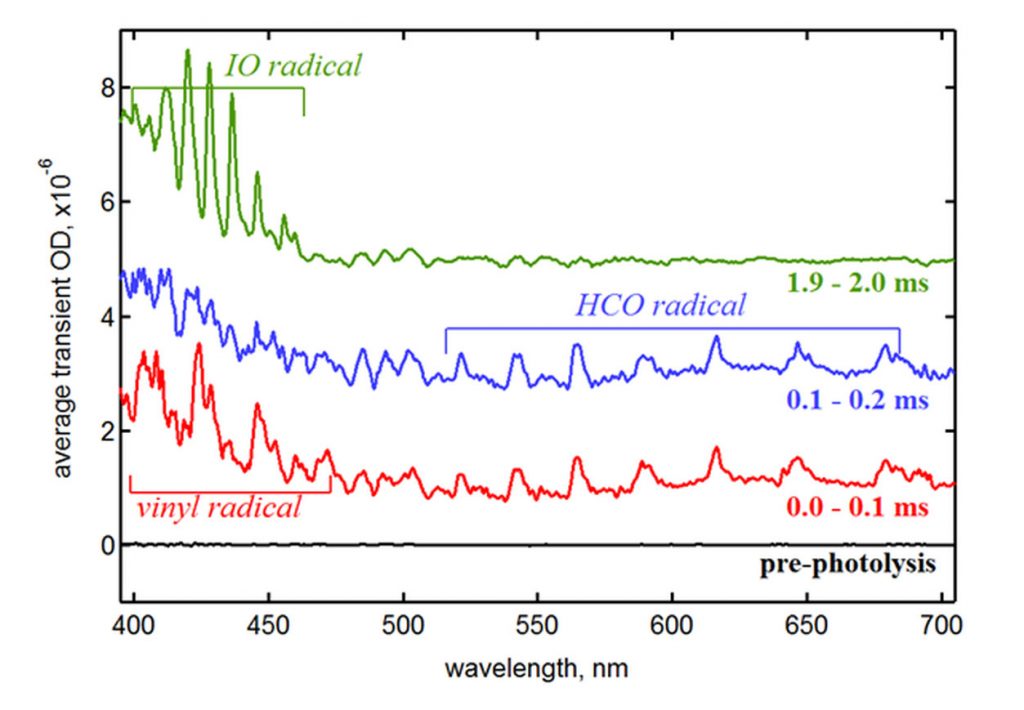
After calibrating his apparatus for wavelength, time, and effective path length, Leonid demonstrated the method’s ability to detect and investigate short-lived reactive intermediates in vinyl radical oxidation, an important combustion process. Figure 2 shows the transient spectra at three times in the reaction of vinyl radicals with O2, including absorption features from the vinyl radical (produced by laser photolysis of vinyl iodide) and formyl radical, which is an intermediate species in vinyl oxidation. In addition, the spectra show absorption due to IO radicals, which are formed by a side reaction of O atoms with an impurity, I2. This example highlights the value of the multiplexed nature of the new method. In experiments using single-color absorption, the interfering and unexpected absorption by IO would produce an experimental artifact that could bias the results or confound researchers. However, with time-resolved broadband detection, the interference is easily identified and corrected for by subtracting its contribution from the spectrum.
“The fact that the system is inexpensive to build means it can be used in small laboratories with limited funding,” Leonid says. He is currently using the TR-BB-CEAS method to characterize Criegee intermediates, a class of chemical species important in combustion and atmospheric science that was directly measured for the first time by researchers from the CRF, the University of Manchester, and Bristol University.
The first publication reporting the UV spectrum of the simplest Criegee intermediate, formaldehyde oxide, was recently published in the Journal of Physical Chemistry Letters.