A central goal of chemistry is to control the path of a chemical reaction, thereby producing a desired product with minimal waste or impurities. However, the arrangement of atoms in any molecule predisposes it to certain reaction pathways, often leading to undesirable products. By pushing molecules far from their equilibrium state, it may be possible to enhance new reaction pathways and inhibit others. For example, we used ultraviolet light to rearrange the atoms in a common molecule (acetaldehyde) into a different, less stable structure (vinyl alcohol).
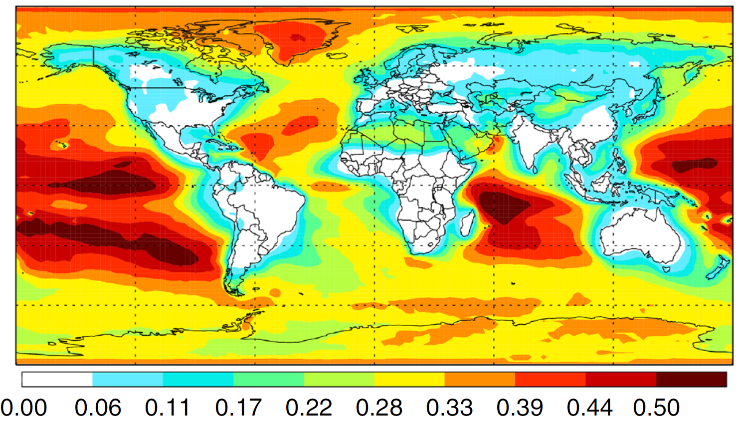
photo-isomerization of acetaldehyde to vinyl alcohol in
GEOS-Chem 3D chemical transport model. Source: Sandia National Laboratories.
In this work, the oxidation product of vinyl alcohol (formic acid) was the desired outcome we hoped to achieve by pushing the system far from equilibrium. However, vinyl alcohol is exceedingly uncommon at thermal equilibrium at room temperature, where there is only 1 vinyl alcohol molecule for every 3.3 million acetaldehyde molecules. Experiments showed that ultraviolet light of the wavelengths that reach the Earth’s surface could alter this equilibrium ratio by more than a factor of 100,000, converting acetaldehyde to vinyl alcohol, and therefore enabling this distinct chemical outcome. In addition to its fundamental value for understanding reaction mechanism control, the research discovered that sunlight can convert acetaldehyde in Earth’s atmosphere into the oxidation product formic acid, which plays an important role in rainwater acidity and is under-predicted by current atmospheric models.
Key Contribution
This far-from-equilibrium process generates up to 60% of total modeled formic acid over mid-latitude oceans on Earth.
Partners
- Balint Szatary, University of the Pacific
- Dwayne Heard, University of Leeds
- Dylan B. Millet, University of Minnesota
- Meredith J. T. Jordan, University of Sydney
- Scott H. Kable, University of New South Wales
PIs: David L. Osborn, Laura McCaslin, David W. Chandler