A collaborative effort between the Gas Phase Chemical Physics program at the Combustion Research Facility and the Chemical Dynamics in the Gas Phase group at Argonne National Laboratory, the consortium program focuses forefront research in gas phase chemical physics on the critical national energy mission of combustion. The program provides the framework for rigorous predictions of combustion chemistry through the design and implementation of novel experiments, theory, and modeling to characterize high-pressure combustion kinetics from elementary reactions, to submechanisms, to flames.
The core of the program’s strategy is to coordinate modeling, experiment, and theory (M-E-T) to accelerate progress in the fundamental understanding of the chemical physics of coupled reactions such as those that drive combustion:
Modeling: Detailed models characterize interrelationships of exothermic reactions that cause emergence of non-equilibrium phenomena
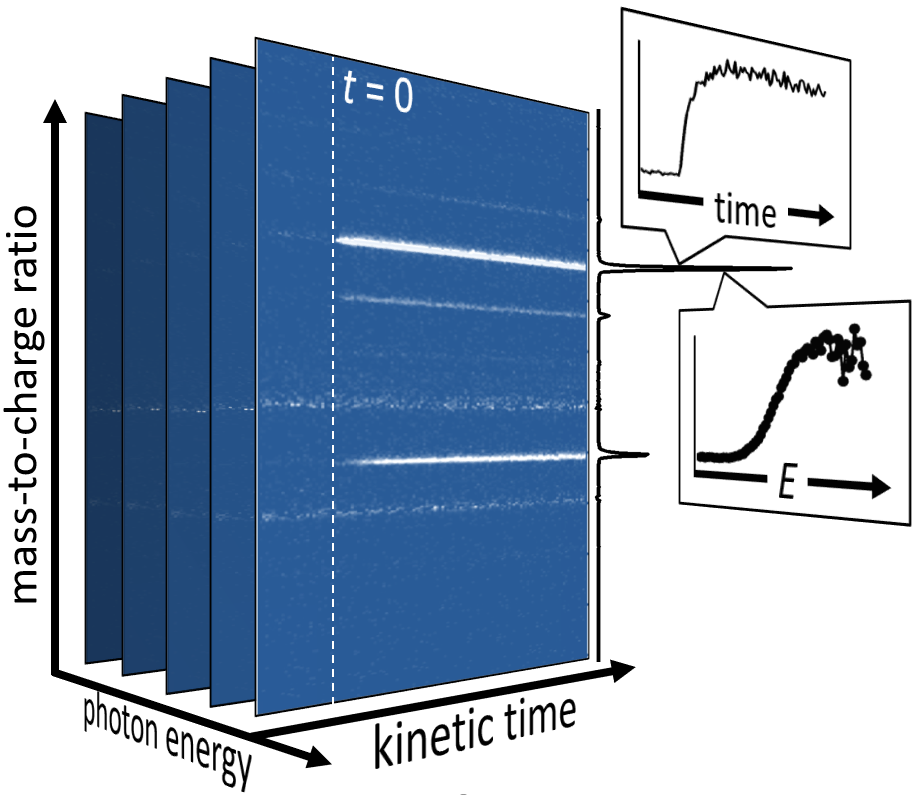
Experiment: High sensitivity measurements quantify kinetics and probe intermediates in elementary reactions and reaction systems.
Theory: First-principles kinetics rigorously characterizes the interplay of energy transfer and reaction.
Key Contributions
- New Insights into Low-Temperature Oxidation of Propane from Synchrotron Photoionization Mass Spectrometry and Multi-Scale Informatics Modeling
- Pressure-Dependent Competition among Reaction Pathways in the Low-Temperature Oxidation of Tetrahydrofuran
- Developing a Theory-Informed Chemical Kinetic Model for the Small-Hydrocarbon Fuels
- Termolecular Chemistry Facilitated by Radical-Radical Recombinations
Partners
Stephen Klippenstein, Robert Tranter, Raghu Sivaramakrishnan, and Ahren Jasper, Argonne National Laboratory
PIs: Leonid Sheps, Craig Taatjes, Nils Hansen, Judit Zador