Technical Details
Oxidation turns hydrocarbons into water and carbon dioxide through an interconnected web of reactions. The hydrocarbon’s molecular structure and the conditions of oxidation, such as temperature, pressure, and composition, profoundly impact phenomena ranging from air quality to engine performance.
In this aspect of our chemical physics program, we focus on low-temperature auto-oxidation. The richness of the chemistry allows us to uncover fundamental physical chemical phenomena while we create quantitative models describing the molecular transformations. Figure 3 shows schematically the major pathways for alkanes, but this scheme is often inaccurate or incomplete for the description of the low-temperature oxidation mechanism of unsaturated or substituted hydrocarbons. We use multiplexed experimental methods to identify and follow the time-evolution of the intermediates in an isomer-resolved manner. We also construct and solve the chemical master equation for these typically multiwell chemical systems. When combined, our theoretical and experimental methods provide a powerful approach to gain fundamental insight into oxidation processes in a wide pressure and temperature range. In addition to understanding fundamental physical chemical concepts, such as structure-reactivity relationships, our research also provides a solid basis for predictive models.
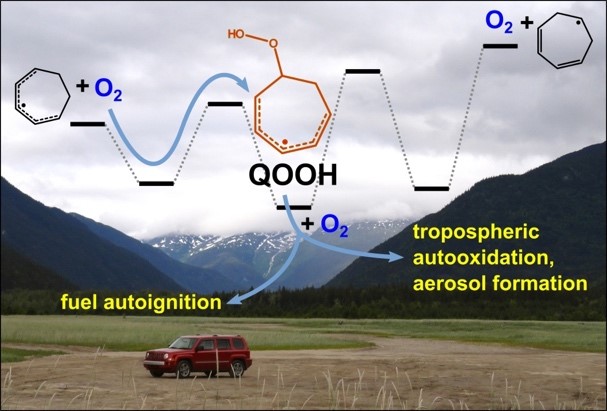
Figure 1 Hydroperoxyalkyl radicals—denoted QOOH—are key intermediates in oxidation chemistry in a wide array of fields. They control autoignition at the early stages of combustion, whereas in the atmosphere they enable rapid autooxidation that leads to the formation of secondary organic aerosols. The Combustion Research Facility plays a lead role in detecting these molecules and predicting their impacts.
Experimental techniques
- Low- and high-pressure photolytically initiated multiplexed photoionization mass spectrometry
- Jet-stirred reactor for thermally initiated multiplexed photoionization mass spectrometry
- Time-resolved step-scan Fourier spectroscopy for state-resolved studies of exothermic reactions
- Time-resolved broadband cavity-enhanced absorption spectroscopy
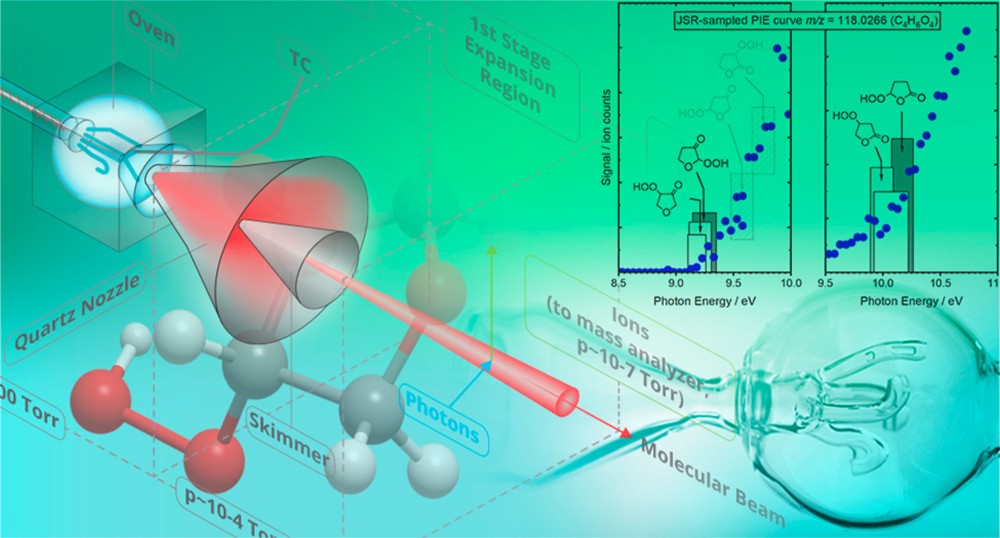
Figure 2 Photoionization mass spectrometry experimentally characterizes key reaction intermediates like ketohydroperoxides, sampled from controlled photolysis or thermal reactors, such as the thermal jet-stirred reactor depicted here. (Source: https://pubs.acs.org/doi/10.1021/acs.jpca.9b07017 )
Theoretical techniques
- RRKM-based master equations for multiwell systems
- Ab initio molecular dynamics
- Automated approaches to construct the potential energy surfaces
Figure 3 General scheme for low-temperature hydrocarbon oxidation. (source: https://www.sciencedirect.com/science/article/pii/S0360128510000559)
Key Contributions
- Detected and characterized ephemeral QOOH species
- Uncovered how O-heterocycles change the course of oxidation
- Discovered unconventional reaction channel in alcohol oxidation
- Interrogating reactive intermediates, including ketohydroperoxides, in diethyl ether oxidation
- Addition of a third O2 molecule in the autooxidation of neo-pentane
- QOOH-mediated oxidation of cyclohexene
- Isomer-specific measurements of ketohydroperoxides
Partners
- Ruben Van de Vijver, University of Ghent
- Stephen J. Klippenstein, Argonne National Laboratory
- Adam Trevitt, University of Wollongong, Australia
- Brandon Rotavera, University of Georgia
- Giovanni Meloni, University of San Francisco
PIs: Nils Hansen, David L. Osborn, Leonid Sheps, Craig A. Taatjes, Judit Zádor