
Over the past two summers and a sabbatical semester in 2013, University of the Pacific (UOP) chemistry professor Balint Sztaray and his research group have been working with the CRF’s David Osborn on a project to improve the selectivity of the current multiplexed gas-phase chemical kinetics instrument. Specifically, the two researchers are focusing on developing a new instrument that combines Balint’s method of photoelectron-photoion coincidence spectroscopy with David’s multiplexed photoionization mass spectrometry (PIMS).
DOE has been a partner in this effort, providing funding to Balint through its Visiting Faculty Program (VFP). Part of the larger Workforce Development for Teachers and Scientists (WDTS) Program run by DOE’s Office of Science, VFP is designed to increase research competitiveness by enabling a college or university faculty member and up to two students to conduct 10 weeks of collaborative research with national laboratory staff.

As a returning VFP award recipient, Balint was back at the CRF this past summer, along with UOP Ph.D. student Kyle Covert, to work with David on bringing their concept closer to reality. Their work to date, which included a trip to Switzerland to run experiments on a unique endstation at the Swiss Light Source (SLS), was described in detail in a proof-of-concept paper published in the prestigious Journal of Physical Chemistry Letters.
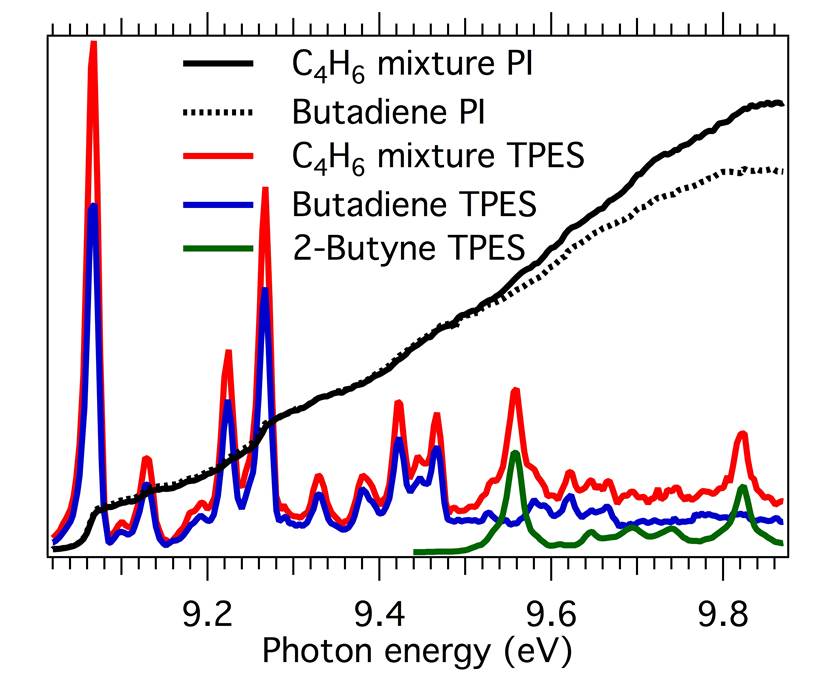
PIMS, which applies tunable synchrotron light to enable isomer separation based on unique photoionization spectra, is a powerful method for time- and space-resolved chemical analysis of reactants, intermediates, and products. However, when three or more isomers are present, identification by photoionization spectra alone can be challenging.
Through experiments at SLS, the researchers showed that imaging photoelectron-photoion coincidence (iPEPICO) spectroscopy—which measures photoelectron spectra corresponding to each cation mass-to-charge (m/z) ratio—provides a more detailed spectral fingerprint than does photoionization spectroscopy. Figure 1 shows the additional information available from the iPEPICO approach.
“David and I built on these preliminary results and, with Kyle’s assistance, designed a testbed experiment to fit into the existing iPEPICO endstation at the VUV [vacuum ultraviolet] beamline of the SLS,” said Balint. The team plans to travel to SLS to test a prototype design. Afterwards, they will design and build a new apparatus for the chemical dynamics beamline of the Advanced Light Source at Lawrence Berkeley National Laboratory, the planned successor of the current very successful multiplex PIMS instrument.
“It was great to have Balint back at the CRF to continue this project,” said David. “And we appreciate DOE’s support of Balint and Kyle through the Visiting Faculty Program. This collaborative work will bring us closer to identifying all the species present in combustion reactions so that the CRF can continue to contribute to cleaner and more efficient engines.”